Clinical Trial Management System (CTMS)
By Optimum Consultancy Services
Accelerate clinical trials with Optimum's CTMS solution. Manage activities, personnel, and compliance. Track issues, dashboards, and training for biotech firms to ensure regulatory adherence and faster market delivery of innovations.

Contact Optimum Consultancy Services
Advance Your Research
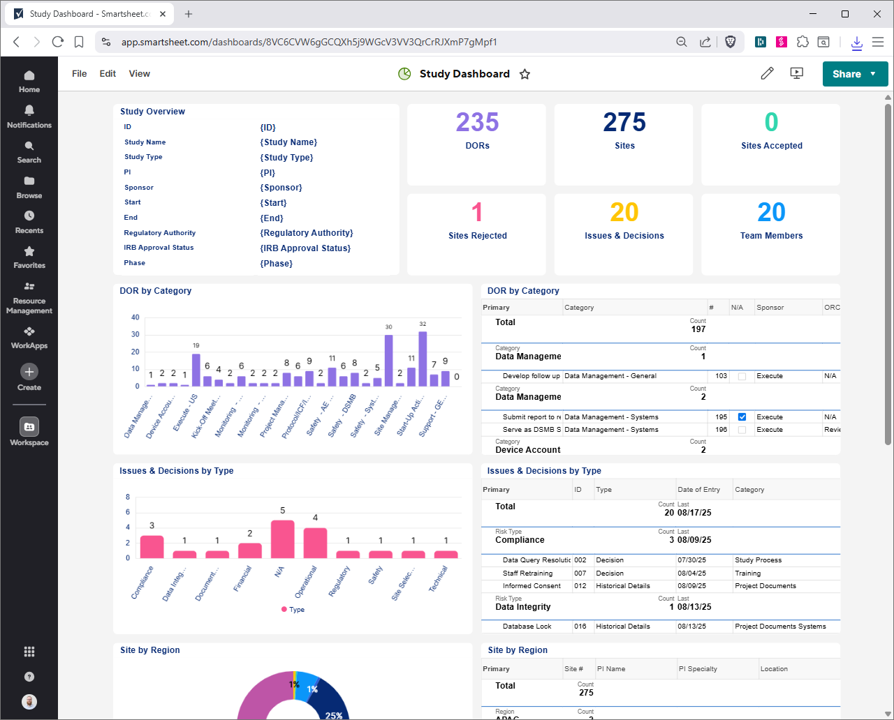
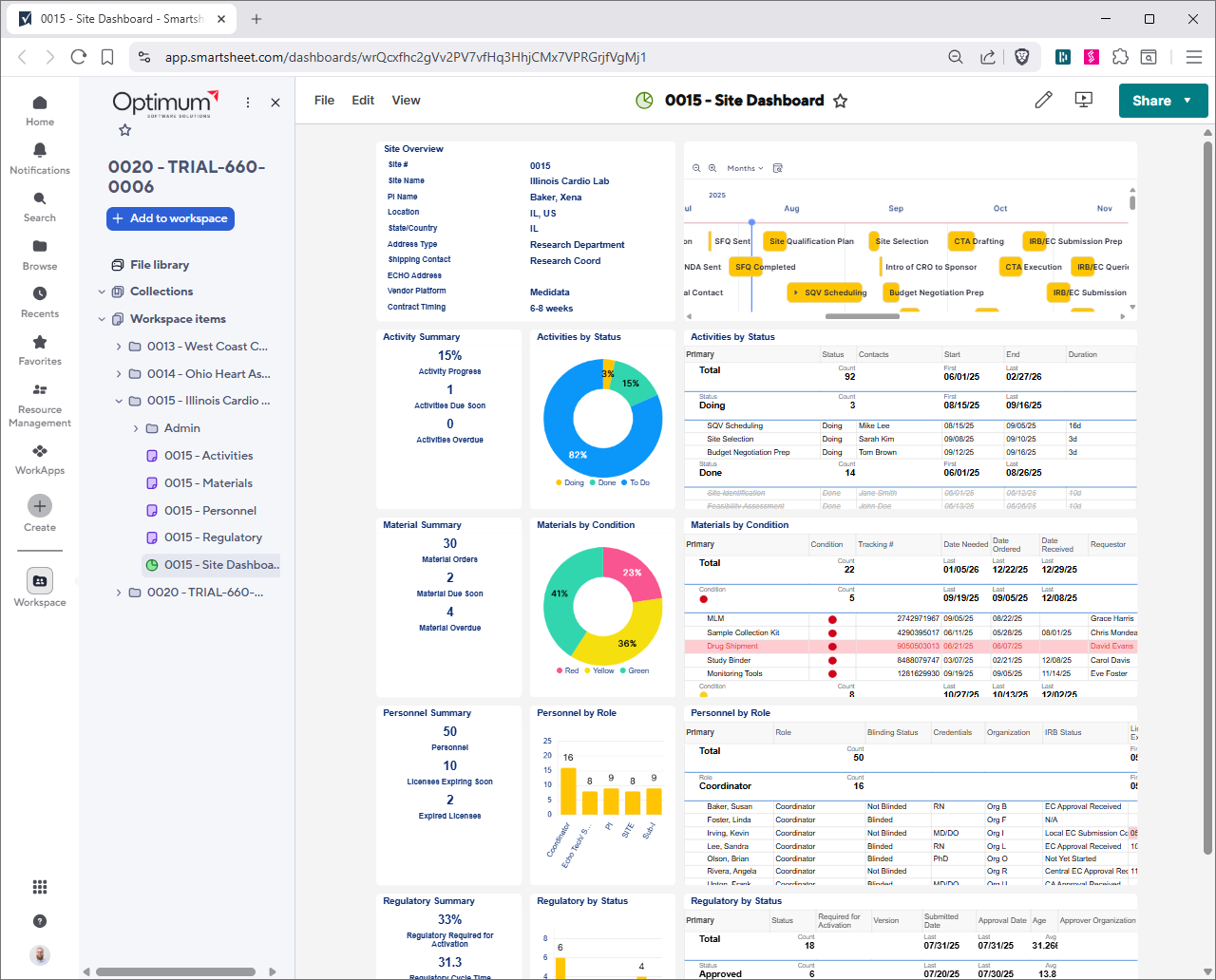
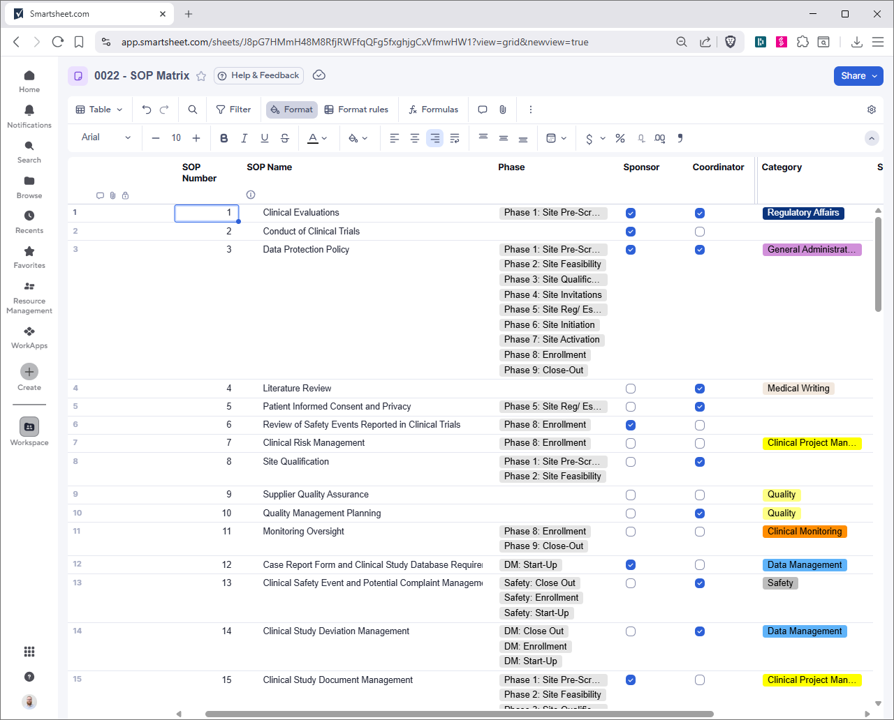
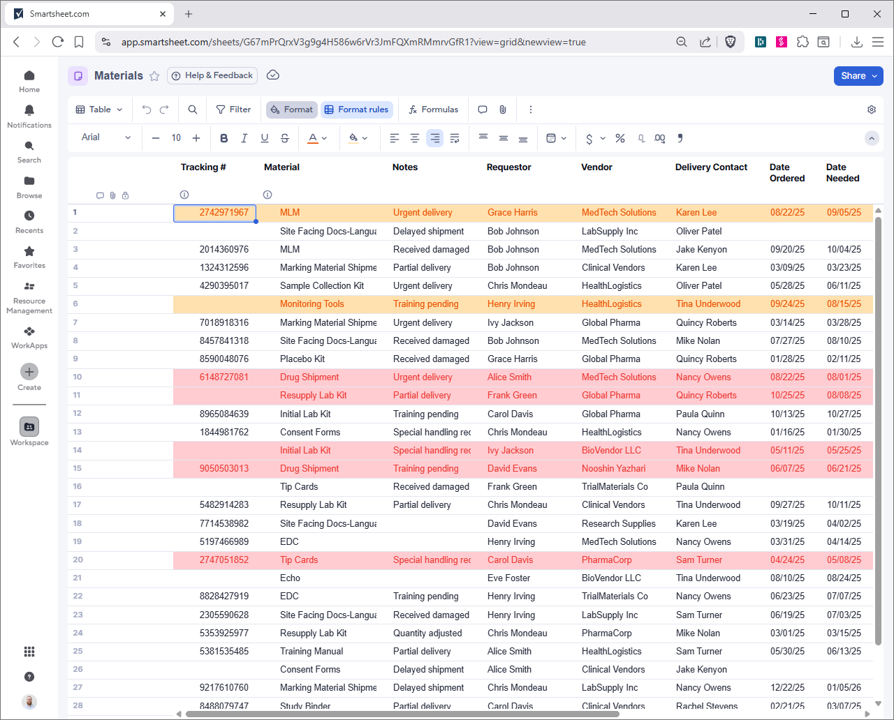
Revolutionize clinical trial management using Smartsheet's CTMS tools. Centralize activities, DORs, and regulatory documents in a secure platform. Monitor issues, materials, and personnel with real-time dashboards for portfolios, sites, and studies. Maintain SOP matrices, team lists, and training logs for compliance. Tailored for biotech, it streamlines workflows, reduces delays, and speeds innovations to market. Customize, integrate, and scale for efficient trial execution.
How to get it:
Contact Optimum Consultancy Services today to explore how to elevate your workflows. Our team will guide you through the setup and configuration to fit your unique business needs.